DMSO-free preservation of cellular therapies and regenerative medicine products
Diverse biological systems (plants, insects, etc.) survive high salt environments, dehydration, drought, freezing temperatures and other stresses through the use of osmolytes. In the human kidney, a mixture of five osmolytes are used to stabilize the cells. We hypothesized that, just as in nature, multicomponent osmolyte solutions (sugars, sugar alcohols and amino acids) can be used to preserve cells effectively. We have confirmed the hypothesis that combinations of sugars, sugar alcohols and amino acids can be effective in preserving cells used therapeutically. The focus of this project is to expand the number of cells that can be stabilized using osmolytes and translating the protocols developed into clinical and commercial application. This includes the production of chemically defined preservation solutions with better and more consistent performance than "home-brew" solutions.
Multicellular systems (aggregates and tissues) exhibit increased sensitivity to freezing. We propose to use strategies developed by nature to stabilize more complex organisms and enhance stability of multicellular systems. This includes exploring Natural Deep Eutectic Systems (NADES) and multicomponent osmolyte solutions for cryogenic and high-subzero storage. The addition of mechanistic preservation molecules, to target cell-specific mechanisms of damage, are being explored and compared to other cell types.
Mechanisms of damage or stabilization during freezing
Damage to cells during freezing comes from a variety of mechanisms. We have established that ice formation inside of cells can be damaging when the fraction of the cell containing ice exceed a critical level. Larger ice crystals are more damaging than small ice crystals. Stabilization of cells during freezing results from both stabilizing critical structures in the cell (i.e. the cell membrane) as well as altering the freezing behavior of water.
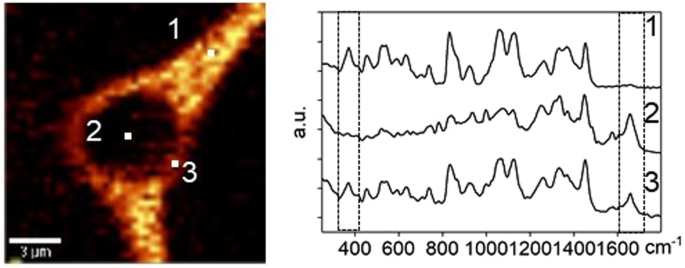
Ongoing research in this area uses low temperature Raman spectroscopy to characterize with great chemical specificity and spatial resolution the response of single cells and tissue to the freezing environment. These techniques are being used to quantify the unique freezing characteristics of cells, nuclei, embryos, and tissues and the manner by which those characteristics result in the greater sensitivity of tissues to freezing. Other techniques to characterize mechanisms of stabilization/damage include differential scanning calorimetry, multiphoton microscopy, and Fourier Transform Infrared (FTIR) Spectroscopy.
The addition of mechanistic preservation molecules, to target cell-specific mechanisms of damage, are being explored and compared to other cell types. This mechanism is distinct from biochemical/osmotic and freezing damage as it is dependent on the cell type and not easily predicted. Molecules, polymers, and other additives are being explored to improve preservation outcomes such as targeting surface protein stabilization, nuclei preservation, mitochondrial function, membrane stabilization, and more. Assays and high-throughput testing are being explored to identify the specific mechanism of all cryoprotective agents and additives.
Streamlining protocol development and integrating automating into preservation protocols
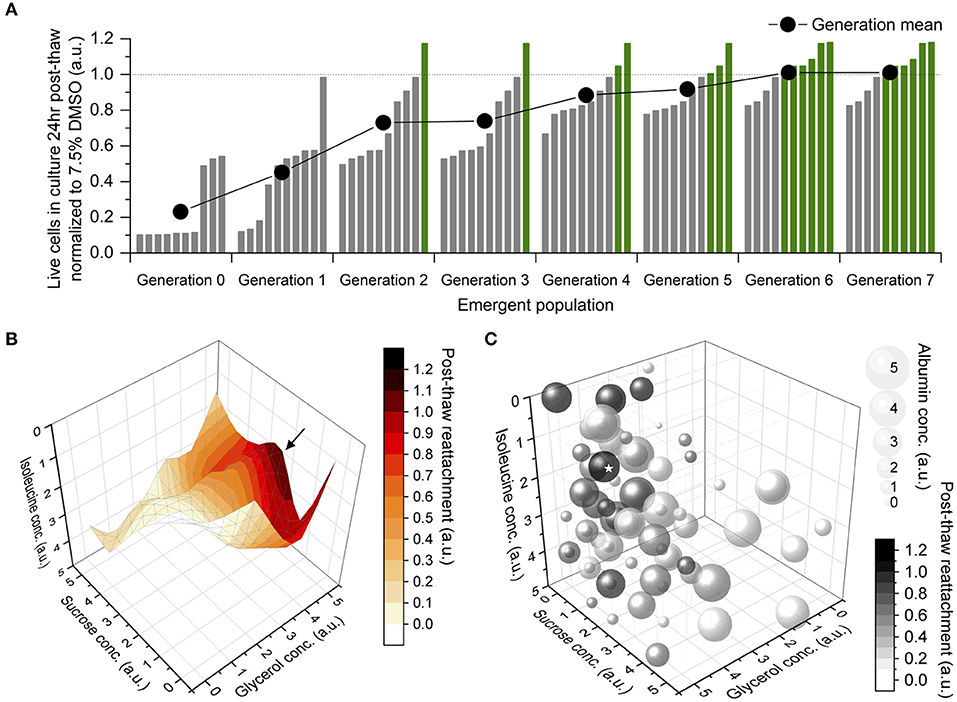
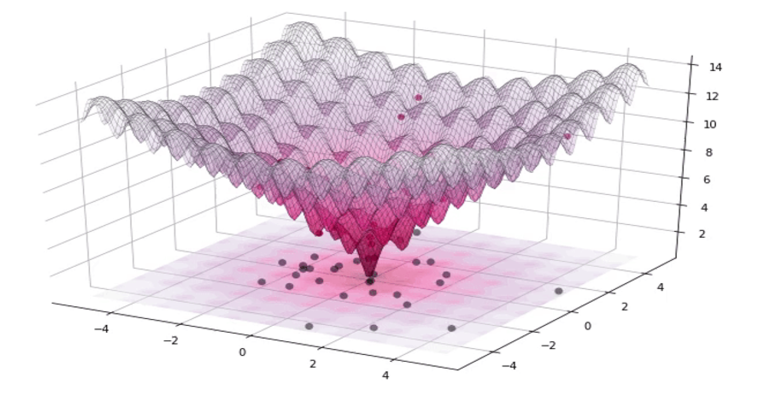
The development of preservation protocols has typically involved selecting a composition and a cooling rate and then measuring the resulting post thaw viability. This process is repeated until an acceptable post thaw recovery is determined. This approach is time consuming and costly. Our approach is to use Differential Evolution to efficiently optimize composition and cooling rate for a given cell type. This approach has enabled us to reduce the number of experiments required for optimization significantly.
Tissues contain a variety of cell types. Effective preservation protocols must result in high levels of post thaw recovery for all of the cell types present in a given tissue or mixed cell population. We are presently applying Differential Evolution with Multiple Objects to efficiently optimize the preservation of multiple cell types.
Preservation protocols are typically labor intensive and there has been little integration of automation into the preservation process. An active area of research involves the integration of robotics/automation into the preservation process. We have demonstrated the ability to use robotic cell culture and manipulation to at both the beginning of the preservation process as well as thawing and post thaw culture of cells. We propose to extend the use of automation to process using high throughput techniques, a large number of biological systems and thereby enhance the throughput for specific systems such as drosophila melanogaster, which require preservation of > 150,000 different lines.